Which Compound Is Formed When Aluminum Bonds With Fluorine
A AIF B AIF C AIF D ALF 12. Aluminum sulfide also called dialuminium trisulfide is an ionic compound of aluminum and sulfur represented by the chemical formula Al2S3 1 2.
This Is How The Ionic Bond Forms In Aluminium Fluoride Alf3 Youtube
See also how do plants make glucose.

. The following fluorine compounds are widely used. Aluminium has 1 valence electrons in their shell and fluorine has 7 valence electrons in their shell. Beryllium is most commonly extracted from the mineral beryl which is either sintered using an extraction agent or melted into a soluble mixture.
Aluminum fluoride uh-LOO-min-um FLOR-ide is a highly stable compound that occurs as a white crystalline solid. Fluorine is more electronegative than chlorine meaning it is better. The sintering process involves mixing beryl with sodium fluorosilicate and soda at 770 C 1420 F to form sodium fluoroberyllate aluminium oxide and silicon dioxide.
Covalent bonds are formed when atoms share electrons and ionic bonds are formed when positive and negative ions bond to form an ionic compound Will fluorine form bonds. 10ー Which pair of elements will most readily form a compound. So the formula of the ionic compound is Al2O3 aluminum oxide.
Aluminum fluoride AlF 3 is used to make pure aluminum from oxidized aluminum. Simple mix aluminum metal or any compound containing aluminum in which aluminum has weak bonds to the other elements with hydrofluoric acid or another acid along with a fluoride salt like LiF or NaF in water to produce in situ HF. An aluminum Al ion has a charge of 3.
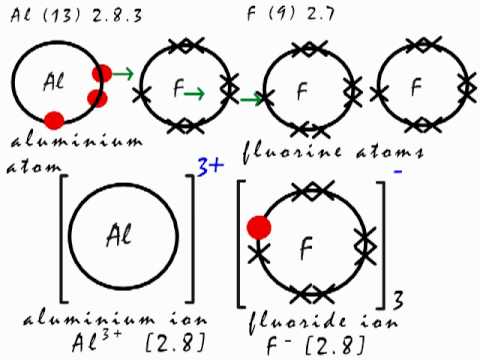
Equal to the charges on the ions. Aluminum belongs to Group 3A so its ion will be Al 3 On the other hand oxygen is in Group 6A making it O 2 Whenever we combine to ions of opposing charges to form a compound we need to exchange their numbers like so. The aluminium donates its three valence electrons to three fluorine atoms and they.
It is an inorganic compound having the formula. Rather than forming 7 bonds fluorine only forms a single bond for basically the same reasons that oxygen only forms two bonds. Aluminum belongs to Group 3A so its ion will be Al3.
A positively charged ion is formed when an atom loses electrons. Whenever we combine to ions of opposing charges to form a compound we need to exchange their numbers like so. Interesting facts about fluorine F.
In terms of electron arrangement in the formation of the ionic compound aluminium fluoride the aluminium donates its three outer electrons to three fluorine atoms forming a triple positive aluminium ion and three single negative fluoride ions. So the formula of the ionic compound is Al 2 O 3 aluminum oxide. In terms of electron arrangement in the formation of the ionic compound aluminium fluoride the aluminium donates its three outer electrons to three fluorine atoms forming a triple positive aluminium ion and three single negative fluoride ions.
Because of the difference in electronegativity of fluorine and chlorine. The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides or into cryolite which plays a key role in aluminium refining. The aluminum compounds in the 3 state are dominantly by covalent chemical bonding but in suitable conditions they formed 2 and 1 compounds like AlCl Al 2 O AlO.
A Li and Ne B Liand H C Li and Be D Li and B Which compound is formed when aluminum bonds with fluorine. Explain how fluorine and aluminum would exchange valence electrons to form an ionic compound. How many ionic bonds can fluorine form.
Is called an anion. Owing to the expense of refining pure fluorine most commercial applications use fluorine compounds with about half of mined fluorite used in steelmaking. Valency of Al is 3 and F is 1 ie.
The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides or into cryolite which plays a key role in aluminium refining. For the complete octet both aluminium and fluorine exchange valence electrons to form an ionic compound. The most common of these hydrates has the chemical formula AlF 3 35H 2 O meaning that for every two molecules of aluminum fluoride in a crystal there are seven molecules of water.
Aluminium and fluorine combines to form aluminium fluoride. Hydrogen fluoride HF has one bond but four centers of electron density around the fluorine. Electronegativity is the measure of how an atom can attract the electrons in a bond.
A fluorine F ion has a charge of -1. The aluminium and fluorine react to give ionic compound aluminium fluoride. On the other hand oxygen is in Group 6A making it O2.
In learning chemistry the tripositive ion Al 3 ions may be stabilized in aqueous solutions through strong hydration energy usually six water molecules held strongly by the. Fluorine gains an electron. Answer 1 of 2.
C is usually a nonmetallic element. It has 9 electrons 2 core and 7 valence. It exists in different crystalline forms and hydrolyzes to aluminum hydroxideoxide 3 4.
It consists of an aluminum atom surrounded by three fluoride atoms connected with covalent bonds. Hydrogen fluoride aluminum fluoride silicon fluorides fluosulfonic acid a solvent catalyst reagent for the preparation of organic compounds containing theSO2F group BF3 a catalyst and fluorocarbons. Then write the compounds chemical formula.

Asf3 Lewis Structure Arsenic Trifluoride Lewis Molecules Electrons

C2h6 Lewis Structure Ethane Math Lewis Chemical Formula

Aluminum Fluoride Formula Structure Properties Manufacturing Study Com
No comments for "Which Compound Is Formed When Aluminum Bonds With Fluorine"
Post a Comment